al molar weight|relative atomic mass vs molar : Tagatay Aluminium (Aluminum in North American English) is a chemical element; it has symbol Al and atomic number 13. Aluminium has a density lower than that of other common metals, about one-third that of steel. It has a great affinity towards oxygen, forming a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to r. Resultado da A Porta de Entrada do Excluído. School Life / Romance . Quem diria que entrar em um clube só de garotas poderia mudar a vida de um excluído .
0 · relative atomic mass vs molar
1 · molar mass of al 3
2 · mass of one mole al
3 · atomic weight vs molar mass
4 · atomic vs molecular mass
5 · alum formula and molar mass
6 · More
7 · 1 mole of aluminum
8 · 1 mol of al
webAlameda Verona, 158 - Pituba Solicitar Informações sobre preços e planos. Marcar aula gratuita ou tirar dúvidas. Outras Unidades. São Caetano. Estrada de Campinas, 494 - São Caetano. Pituba. Rua Miguel Navarro .
al molar weight*******Element Aluminium (Al), Group 13, Atomic Number 13, p-block, Mass 26.982. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content . Periodic Table. Home; . Relative atomic mass The mass of an atom relative .Al (Aluminium/Aluminum) 26.9815386. 3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get .Aluminium (Aluminum in North American English) is a chemical element; it has symbol Al and atomic number 13. Aluminium has a density lower than that of other common metals, about one-third that of steel. It has a great affinity towards oxygen, forming a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to r. Learn how to calculate the molar mass of aluminum from its atomic weight on the periodic table. The molar mass of aluminum is 26.982 g/mol.Aluminum. Formula: Al. Molecular weight: 26.9815386. IUPAC Standard InChI:InChI=1S/Al Copy. IUPAC Standard InChIKey:XAGFODPZIPBFFR-UHFFFAOYSA-N Copy. CAS .Symbol: Al Atomic Mass: 26.981538 # of Atoms: 1 Mass Percent: 100.000%. Calculate the molecular weight of a chemical compound. Enter a chemical formula: Browse the list of .Aluminum is the 13th element in the periodic table and has a symbol of Al and atomic number of 13. It has an atomic weight of 26.98154 and a mass number of 27. . Find the molar masses of carbon (C), hydrogen (H), and oxygen (O). Count the number of atoms of each element in the compound. Find the molar mass of glucose .Atomic # Molar Mass Total Mass; Al: 1: Aluminum: 13: 26.981: 26.981: Total Mass : 26.981 : The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our .al molar weight relative atomic mass vs molar Find the molar masses of carbon (C), hydrogen (H), and oxygen (O). Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the .
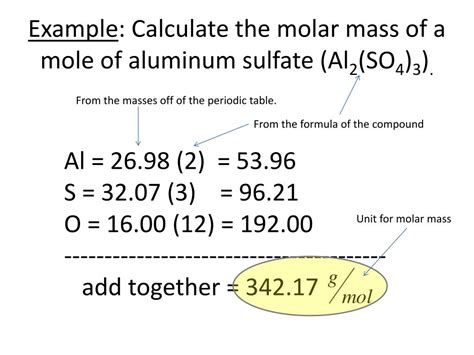
One mole of Al atoms has a mass in grams that is numerically equivalent to the atomic mass of aluminum. The periodic table shows that the atomic mass (rounded to two decimal points) of Al is 26.98, so 1 mol of Al atoms has a mass of 26.98 g. According to the periodic table, 1 mol of U has a mass of 238.0 g, so the mass of 2 mol is twice that .

Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure. Element Groups: . Symbol: Al Atomic Number: 13 Atomic Mass: 26.981539 amu Melting Point: 660.37 °C (933.52 K, 1220.666 °F) Boiling Point: 2467.0 °C (2740.15 K, 4472.6 °F)al molar weightAtomic # Molar Mass Total Mass; Al: 1: Aluminum: 13: 26.981: 26.981: Total Mass : 26.981 : The molar mass of Al (Aluminium) is: 26.981 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). Tool Overview: Molar Mass of Aluminium (Al) 1 mol Al = 26.98 g Al. Prepare a concept map and use the proper conversion factor. Cancel units and calculate. 3.987 molAl × 26.98gAl 1 molAl = 107.6gAl 3.987 mol Al × 26.98 g Al 1 mol Al = 107.6 g Al. Think about your result. The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum.Aluminum (Al) Atomic Data for Aluminum (Al) Atomic Number = 13 Atomic Weight = 26.98154 Reference E95 : Isotope : Mass : Abundance : Spin : Mag Moment : 27 Al: 26.981540: 100%: 5/2 +3.6415: Al I Ground State 1s 2 2s 2 2p 6 3s 2 3p 2 P 1 / 2 Ionization energy 48278.48 cm-1 (5.985768 eV) Ref. KM91bMolar mass of Al (Aluminium) is 26.98153860 ± 0.00000080 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between Al weight and moles. Compound.relative atomic mass vs molarThe mass of 1 mol of NaCl is 58.45 g. Multiplying the molar mass of each atom by the number of atoms of that type in bilirubin’s formula and adding the results, we get. 33 C molar mass: 33 × 12.01 g. 396.33 g. 36 H molar mass: 36 .Atomic and nuclear properties of aluminum (Al) Quantity: Value: Units: Value: Units: Atomic number: 13 : Atomic mass: 26.9815385(7) g mol-1 : Density: 2.699 : g cm-3 : Mean excitation energy: 166.0: eV : Minimum ionization: 1.615: MeV g-1 cm 2: 4.358 . x ray mass attenuation coefficients from NIST .Calculando a massa molar passo a passo; Primeiro, calcule o número de cada átomo em Al(aluminio): Al: 1. Em seguida, pesquise os pesos atômicos para cada elemento da tabela periódica: Al: 26.9815386 Agora, calcule a soma dos produtos do número de átomos pelo peso atômico: Massa molar (Al(aluminio)) = ∑ Count i * Weight i = Count(Al .Aluminum is the 13th element in the periodic table and has a symbol of Al and atomic number of 13. It has an atomic weight of 26.98154 and a mass number of 27. Aluminum has thirteen protons and fourteen neutrons in its nucleus, and thirteen electrons in three shells. It is located in group thirteen, period three and block p of the periodic .
WEBAcione Compra Segura. Acione seu Seguro. Você deseja acionar uma proteção do seguro Compra Segura? Para isso, selecione a proteção desejada, confira a documentação .
al molar weight|relative atomic mass vs molar